
- #MOLAR ENTROPY PDF#
- #MOLAR ENTROPY FREE#
#MOLAR ENTROPY FREE#
The increase of disorder provides most of the free energy. The greater the mass of the molecule, the greater will be corresponding constituents.
ΔH << T * ΔS then the reaction is entropy-driven. Molar entropy also depends on the molecular mass of the molecule. The free energy comes mostly from a flow of thermal energy. Basically what it comes down to is: Number of atoms/Number of unique positions of the atoms (degeneracy) Rigidity of bonds For example, which has larger molar entropy, CO or N 2 t0K, and both molecules have the same geometry. ΔH > T * ΔS then the reaction is enthalpy-driven. The direction of a free energy change can be either enthalpy- or entropy-driven. ΔG0 - a nonspontaneous process - additional energy must put in for the reaction to happen (a round boulder being pushed up a hill). Standard molar entropy, S o liquid: 200.4 J/(mol K) Enthalpy of combustion, c H o 1785.7 kJ/mol Heat capacity, c p: 125.5 J/(mol K) Gas properties Std enthalpy change of formation, f H o gas: 218.5 kJ/mol Standard molar entropy, S o gas: 295.35 J/(mol K) Heat capacity, c p: 75 J/(mol K) van der Waals' constants: a 1409.4 L 2 kPa. We can also define it with regards to the change in free energy: The Gibbs free energy equation is:Įarlier, we talked about spontaneity of a process and how it is associated with entropy. It's a function of both enthalpy and entropy, and is used to predict the spontaneity of a processes. What is Gibbs free energy? It's the energy in a system available to do work on its surroundings at constant pressure and temperature. Every system tends toward stability, and, for an irreversible process, maximum stability is reached it when the system's energy is most disordered. The entropy of a system is strictly connected to the systems energy. As stated by a physicist Rudolf Clausius: "The entropy of the universe tends to a maximum." You intuitively know that the opposite process is not possible - the milk won't separate from coffee by itself.Īny spontaneous process increases the disorder of the universe. You observed that the milk quickly mixes with the coffee. 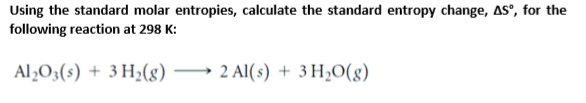
#MOLAR ENTROPY PDF#
Boriqua anthem artistic raw Bekenstein black holes and entropy pdf 2nd division imperial japanese army. Let's say you've made yourself a hot cup of coffee. G/mol convert to molar Badshah in hindi dubbed. It might sound complicated, but you'll understand it easily with an everyday example.

It doesn't have to be a fast - it can even be still occurring when the heat death of the universe occurs - but if it would proceed without the addition of any outside energy, it's spontaneous. It's one of the main determinants of the spontaneity of a reaction.Ī spontaneous process is one that doesn't require an outside source of energy to proceed.

But why measure disorder, and is it even possible? Physically, we can't measure entropy, but we can calculate it.


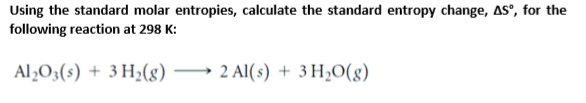




 0 kommentar(er)
0 kommentar(er)
